


Projects from the Hidalgo lab


Glia maintain follower neuron survival in the Drosophila CNS
Neurons are produced in excess during development of the mammalian nervous system and brain, and excess cells are subsequently eliminated, adjusting innervation to functional requirements. In Drosophila, many neurons are also eliminated during development. We asked whether neuronal survival in fruit-flies is also adjusted in a plastic manner. We used genetic methods to ablate glial cells only, and found that this increased neuronal death over normal levels. Furthermore, we could rescue neuronal death if we also expressed a gene that prevents cell death or if we ablated the glia in mutant specimens that lacked apoptosis. Our findings demonstrated for the first time that neuronal number is not fixed in flies and it is instead adjusted through cell interactions. This led us to search for neurotrophic and gliatrophic factors in Drosophila.
Funded by: The Wellcome Trust.
See: Booth et al 2000 Development and our reviews, eg Hidalgo 2002 TINS.
Axon guidance: glial cells influence the neuronal Robo code
The Robo code is the molecular demonstration of the selective fasciculation hypothesis. A gradient of a chemorepellant known as Slit is produced by midline glial cells and interneurons that cross the midline sense it, and project away from the midline, never to cross it again. We wondered what function longitudinal glial cells, which are located away from and parallel to the midline, might have in the reading of the Robo code. We showed that the Robo receptor is expressed by longitudinal glial cells prior to axon guidance, to guide glial migration and positioning parallel to the midline. We demonstrated that there are synergistic interactions between the glial gene gcm, trophic support and robo signalling to locate the trajectories of axons. In the absence of trophic pressure and gcm, axons cross the midline ignoring the repulsive Robo code. In embryos that are mutant for both gcm and robo, axons reproduce phenotypes caused by the loss of multiple robo-code axonal receptors. These findings show that glial cells have essential functions in axon guidance, and that the control of neuronal survival, glia and the Robo code function in concert to establish axonal networks.
Funded by: BBSRC studentship and The Wellcome Trust.
See: Kinrade et al 2001; Kinrade et al 2004.
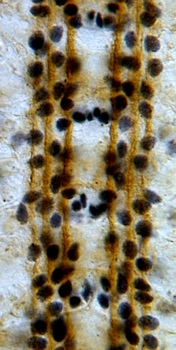
Neuregulin and PVF have conserved functions as gliatrophic factors:
We have shown that the control of neuronal and glial cell survival is a more ancient mechanism than previously thought, conferring plasticity to nervous system development also in invertebrates. Two of the main gliatrophic factors in vertebrates are Neuregulin and PDGF. Drosophila glia number is regulated by the non-autonomous control of cell survival, using these conserved molecular pathways: (1) Targeted neuronal ablation results in an increase in glial apoptosis. The Drosophila Neuregulin homologue, called Vein, is produced by neurons and regulates the survival of a subset of the longitudinal glia via the Ras/MAPKinase/ERK pathway. This was the first evidence of a conserved gliatrophic factor from vertebrates to flies. (2) The PVF/PVR signalling pathway regulates midline glial survival and migration in Drosophila, via the P13K/Akt pathway. This is likely to be redundant with the EGF pathway, which has been well documented by others to promote midline glia survival. The effects on cell number can be more subtle in fruit-flies compared to vertebrates, but the mechanisms are conserved and influence axon guidance.
Funded by: MRC studentship, The Wellcome Trust, MRC CEG and EMBO YIP.
See Learte et al 2008; Hidalgo et al 2001; Kinrade et al 2001; and several reviews.
Glial cells guide pioneer neuron axon guidance and fasciculation
Glia had long been proposed to function as guidepost cells during axon guidance. We tested this idea in Drosophila, using targeted genetic ablation of very small numbers of glial cells at a time, and looking also at mutants lacking the gcm gene which is necessary for normal glial development. We showed that in the central nervous system (CNS) longitudinal glial cells migrate together with but ahead of the extending growth cones, and project large lamellipodia that explore the territory ahead of the growth cones. Glia occupy choice positions that guide axon guidance decisions by pioneer neurons. Targeted genetic ablation of glial cells alters axonal fasciculation and growth cone steering at choice points. Defects in glial cells upon loss of the glial gene gcm also results in axon guidance defects. We thus demonstrated that glial cells play important roles during axon guidance, orienting growth cones and triggering fasciculation and defasciculation decisions at choice points.
Funded by: The Wellcome Trust.
See Hidalgo and Booth 2000; Kinrade et al 2001; Learte & Hidalgo 2007.
Neuron-glia interactions during axon guidance:
neurotrophic and gliatrophic factors in Drosophila


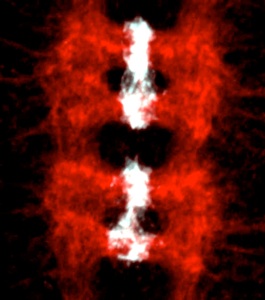
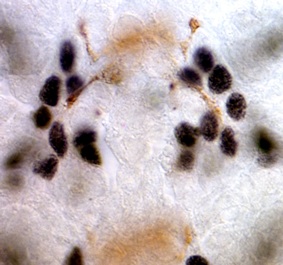
Drosophila
neurotrophins



