



Projects from the Hidalgo lab


Control of glial proliferation is linked to neuronal circuit formation
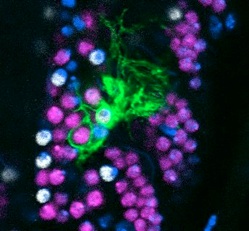
We have shown that the control of glial cell proliferation is coordinated with neural circuit formation. Glial cells are delivered sequentially, during circuit formation. At these time points, glial cells function as guidepost cells, orienting growth cone guidance and axon fasciculation. The controlled delivery of glial cells sorts axonal patterns through time. Ablation of neurons causes a drop in glial division first, and an excess in glial proliferation later on, at times when glia do not normally divide. This latter over-proliferation mimics the repair response of vertebrate glia upon neuronal injury.
The gene prospero couples glial proliferation to axon guidance. Prospero is present in all dividing longitudinal glia during axon guidance and subsequently in glia that remain as immature precursors with mitotic potential. Notch is also activated in the longidutinal glia that have Prospero. Prospero and Notch maintain each other’s expression in these glial cells. Some of the longitudinal glia switch on Numb, the antagonist of Notch, and also switch off Prospero, and exit the cell cycle. Pros+ Notch+ glia instead remain arrested in the G1 phase. This G1 quiescence enables glial cells to respond to interactions to neurons, that can lead to number adjustments in response to limited environmental changes during development. In normal development, the tempo of glial cell division depends on interactions with axons.
Regeneration and repair in the central nervous system
in flies and mice: glia, genes, mechanisms.
Damage to the CNS (e.g. brain and spinal cord) induces the proliferation of glial cells and spontaneously re-enwrapment of axons, revealing a natural tendency of the CNS to repair itself. This is known as the ‘glial regenerative response’ and it is present from cockroaches and flies, to fish and humans. We recently discovered a gene network in Drosophila that regulates the regenerative ability of glial cells in response to injury.
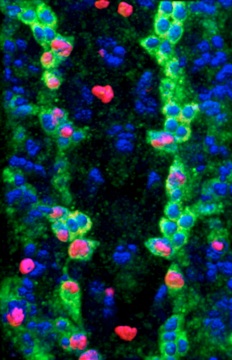
For this, we first established a novel injury paradigm in the Drosophila larval ventral nerve cord. We demonstrated that stabbing injury induces glial proliferation, apoptosis, vacuolisation, activation of enwrapping glia which become phagocytic and engulf cellular debris, and some extent of injury repair also in wild-type, as observed with time-lapse microscopy. These events underlie the macroscopic progression of the lesion from initial vacuolisation and enlargement, to clearance and repair.
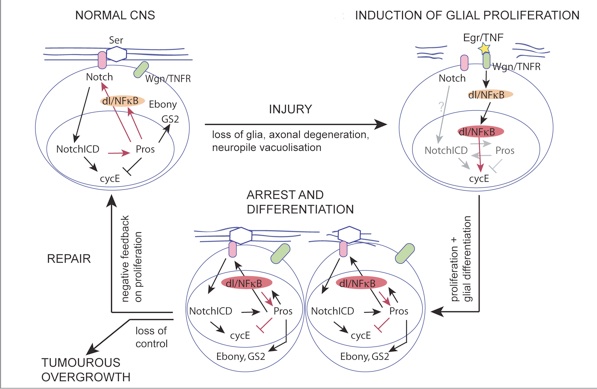
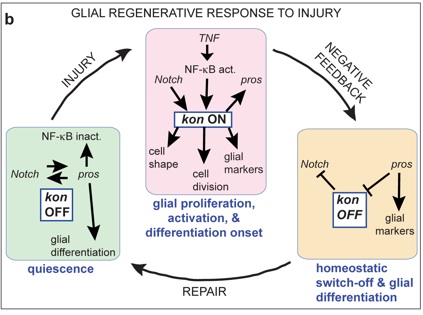
We discovered a gene network that underlies this endogenous regenerative response and can be manipulated to induce repair. The gene network comprises two feedback loops, one involving the genes Prospero (Pros) and Notch, and the second one involving Pros and NFκB. This gene network controls the balance between glial proliferation and glial differentiation. It enables glia to divide upon injury, restore arrest preventing uncontrolled proliferation, and differentiate. It has homeostatic properties as two cell cycle activators (Notch and NFκB) promote the expression of a cell cycle inhibitor (Pros), providing negative feedback on cell division. Pros is also essential for glial differentiation, enabling debris clearance and axonal enwrapment, and priming glia for further responses. By removing these genes or adding them in excess, we shifted from prevention to promotion of lesion repair. In normal development and adult life, this gene network is a homeostatic mechanism for structural robustness.
The Prospero-Notch gene network enables the glial regenerative response to CNS injury


Griffiths & Hidalgo 2004 EMBO J;
Hidalgo & Griffiths 2004 Cell Cycle;
Griffiths et al 2007 Neuron Glia Biology.
In collaboration with mammalian experts Prof. Ann Logan (IBR, Medical School, University of Birmingham) and Dr Fumio Matsuzaki (Center for Developmental Biology, RIKEN, Japan), we asked whether this network might be evolutionarily conserved in the mouse.
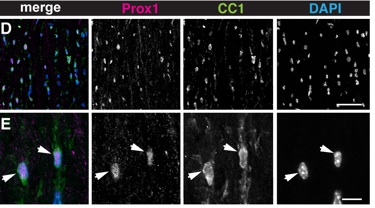
We demonstrated that Prox1 is expressed in Notch1+ NG2+ Oligodendrocyte Progenitor Cells (OPCs) and at higher levels in all Oligodendrocytes (OLs). Using siRNA and conditional prox-1 knock-out in the OPC/OL cell lineage, we showed that Prox1 inhibits proliferation in OPCs and is required for OL differentiation.
The genetic mechanism suggests that this gene network may have been conserved in evolution, as a mechanism to promote structural homeostasis and robustness.
Investigating the involvement of Prox1 in OL differentiation, merits closer attention.
Evolutionarily conserved function of Prox1 inhibits oligodendrocyte progenitor proliferation and promotes oligodendrocyte differentiation in the mouse


See our papers:
doi: 10.3791/50306.

Molecular mechanism of central nervous system repair by the Drosophila NG2 homologue kon-tiki
Back in the fly, we showed that the Drosophila NG2 homologue kon-tiki (kon) is functionally linked to the Pros-Notch gene network.
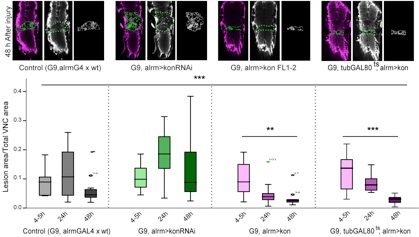
We showed that kon is required for glial proliferation and onset of differentiation in development, and it is switched off in differentiated cells. Kon is activated in injury, where it controls the glial regenerative response, enabling glial proliferation and differentiation onset. We demonstrated that Kon is functionally linked to Pros and Notch.
We showed that by manipulating Kon levels we could prevent or promote central nervous system injury repair.
Our data show that an evolutionarily conserved gene network involving Notch, Kon/NG2 and Pros/Prox1 enables the glial regenerative response to CNS injury.



Drosophila
neurotrophins




