


Projects at the Hidalgo lab


Tolls as neurotrophic receptors:
novel mechanism of cell number plasticity in the CNS.
Toll-6 and Toll-7 function as neurotrophin receptors in the Drosophila CNS
Mammalian neurotrophins exert their functions by binding to receptors of the Trk and p75 families, and activate signalling downstream to promote cell survival (via ERK, AKT and NFkB) or cell death (via JNK) and synaptic plasticity (PLCgamma, CREB).
Neurotrophin receptors of the canonical Trk or p75 protein families have not been found in Drosophila, provoking the question of how is neurotrophism implemented in insects. The neurotrophin family members in Drosophila are DNT1, DNT2 and Spz, which share sequence, structure and functional conservation with the mammalian neurotrophins. Spz is well-known ligand of Toll. Toll was first discovered in Drosophila for its function in establishing the embryonic dorso-ventral axis, and later found to have functions also in innate immunity. Mammalian Toll-Like Receptors (TLRs) were subsequently discovered by homology to Drosophila Toll, and found to also have functions in innate immunity, and alterations in their function results in multiple diseases - from inflammation and autoimmunity to kidney disease. The Toll receptor superfamily has universal functions in innate immunity throughout the animals.


We found that DNT1 and DNT2 bind Toll-6 and Toll-7 receptors in Drosophila and have functions in the central nervous system (CNS) at all stages, from the embryo to the adult brain. They are expressed in CNS interneurons and motoneurons. Loss of Toll-6 and Toll-7 function results in impaired larval locomotion and motoraxon targeting defects. Over-expression of Toll-6 or Toll-7 also impairs motoraxon targeting. The incidence of apoptosis in the CNS increases in Toll-6 or Toll-7 mutants, and apoptosis leads to cell loss, at least amongst interneurons. Over-expression of Toll-6 and Toll-7 can rescue naturally occurring cell death in the CNS.
DNT1 and DNT2 interact genetically with Toll-6 and Toll-7, suggesting a preferential interaction of Toll-7 with DNT1, and Toll-6 with DNT2. These interactions are likely to be promiscuous. Over-expression of Toll-6 and Toll-7 in neurons rescues the apoptosis increase of DNT2 and DNT1 mutants, respectively.
In collaboration with Prof. Nick J. Gay and his team at the Department of Biochemistry, University of Cambridge, we have shown that DNT1 and DNT2 can bind Toll-6 and Toll-7 in vitro, in cell culture and in vivo and one of the downstream targets of Toll-6 and Toll-7 is NFkB. Interestingly, NFKB is also the downstream target of p75, the universal and most ancient of the neurotrophin receptors.
Thus, Toll-6 and Toll-7 function as receptors for DNT2 and DNT1.
Our findings suggest that either neurotrophism is implemented by a different mechanism in flies compared to humans, or that there might be a previously unforeseen relationship between the neurotrophin and TLR families also in mammals. In either case, these findings are important in order to continue using Drosophila to understand the CNS and brain, and to model brain diseases.
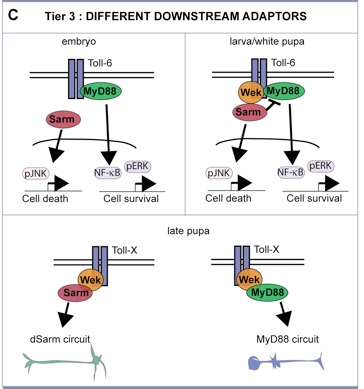
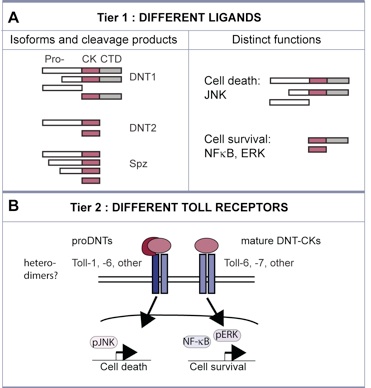


We found a novel mechanism regulating cell number plasticity in the CNS by DNTs and Tolls, and that operates at three levels:
-
- Tier 1: distinct DNTs can regulate either neuronal survival or death, depending on whether they are
received in pro-DNT or cleaved form. DNT1 is more likely to be found in pro- form than DNT2 in vivo.
-
- Tier 2: there are nine Toll receptors, and they can have distinct cellular outcomes. In our assays, Toll-1
was more likely to induce apoptosis than Toll-6.
-
- Tier 3: three intracellular adaptors can shift the response of Tolls between cell survival and death.
MyD88-NFkB induced cell survival; Sarm-JNK induced death; and Wek functions like a hinge to link both pathways downstream of Tolls, but is absolutely required for the pro-apoptotic function via Sarm-JNK.
This novel mechanism of cell number plasticity could also operate in the mammalian brain or in wider contexts.
Foldi, Anthoney et al (2017) Three tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila. J Cell Biol
selected as “One of the Top 10 articles of 2017”
see also review of our findings by Keeler & Deppmann (2017) J Cell Biol.
Three tier regulation of cell number plasticity by neurotrophins and Tolls in Drosophila
Drosophila
neurotrophins



