Projects at the Hidalgo lab

Keks are kinase-less Trk-like receptors:
novel mechanism of structural synaptic plasticity

Neurotrophism, structural plasticity, learning and long-term memory in mammals critically depend on neurotrophins binding Trk receptors to activate tyrosine kinase (TyrK) signaling, but Drosophila lacks full-length Trks, raising the question of how these processes occur in the fly. Trk receptors had long been sought in Drosophila a not found. Pervious searches focused on aiming to identify a conserved tyrosine kinase domain of the Trk family - as all important Trk functions were thought to depend on its kinase function. These searches failed to find an orthologue, and subsequent proteomic analyses confirmed there are no full-length Trk receptors in Drosophila.
Paradoxically, truncated Trk isoforms lacking the TyrK predominate in the adult human brain, but whether they have neuronal functions independently of full-length Trks is unknown.
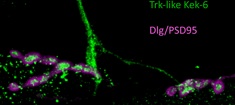
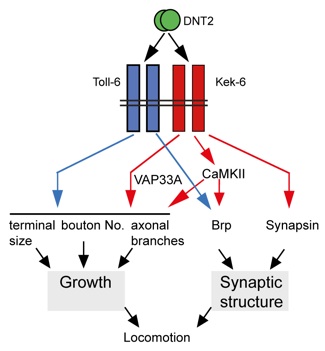
Drosophila has TyrK-less Trk-family receptors, encoded by the kekkon (kek) genes, suggesting that evolutionarily conserved functions for this receptor class may exist. Here, we asked whether Keks function together with Drosophila neurotrophins (DNTs) at the larval glutamatergic neuromuscular junction (NMJ). We tested the eleven LRR and Ig-containing (LIG) proteins encoded in the Drosophila genome for expression in the central nervous system (CNS) and potential interaction with DNTs. Kek-6 is expressed in the CNS, interacts genetically with DNTs and can bind DNT2 in signaling assays and co-immunoprecipitations. Ligand binding is promiscuous, as Kek-6 can also bind DNT1, and Kek-2 and Kek-5 can also bind DNT2. In vivo, Kek-6 is found presynaptically in motoneurons, and DNT2 is produced by the muscle to function as a retrograde factor at the NMJ. Kek-6 and DNT2 regulate NMJ growth and synaptic structure. Evidence indicates that Kek-6 does not antagonise the alternative DNT2 receptor Toll-6. Instead, Kek-6 and Toll-6 interact physically, and together regulate structural synaptic plasticity and homeostasis. Using pull-down assays, we identified and validated CaMKII and VAP33A as intracellular partners of Kek-6, and show that they regulate NMJ growth and active zone formation downstream of DNT2 and Kek-6. The synaptic functions of Kek-6 could be evolutionarily conserved. This raises the intriguing possibility that a novel mechanism of structural synaptic plasticity involving truncated Trk-family receptors independently of TyrK signaling may also operate in the human brain.
Drosophila
neurotrophins



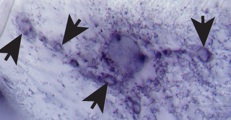
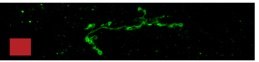
DNT2 mRNA at NMJ synapse
DNT2 expressed in muscle
detected in motor-axons
kek-6 is expressed in motoneurons
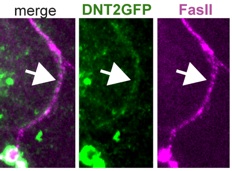
DNT2 is a retrograde ligand for Kek-6 and Toll-6 at the glutamatergic NMJ
•Ulian- Benitez, Bishop, Foldi, Wentzell, Okenwa, Forero, Zhu, Moreira, Phizacklea, McIlroy, Gay, Hidalgo (2017) Kek-6: a truncated Trk-like receptor for Drosophila Neurotrophin 2 regulates structural synaptic plasticity. PLoS Genetics 13(8): e1006968

DNT2 and Kek-6 are required for
NMJ growth and synaptic boutons
wt
DNT2 loss of function mutant
kek-6 loss of function mutant
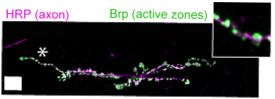
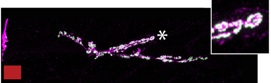
Kek-6 and Toll-6 function cooperatively at the NMJ to regulate synaptic structural plasticity
Kek-6 function activates CaMKII
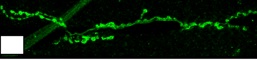
kek-6 loss of function mutant
At the glutamatergic neuromuscular junction (NMJ),
DNT2 is a retrograde factor for its ligands Kek-6 and Toll-6, both localised pre-synaptically in the motoneuron. Kek-6 function activates CaMKII, although how this comes about is unknown. Kek-6 and Toll-6 function cooperatively to regulate NMJ growth, synaptic boutons and synaptic structure. Furthermore, if both are lost, compensatory mechanisms that regulate homeostasis at the synapse, fail.
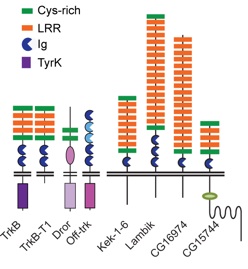
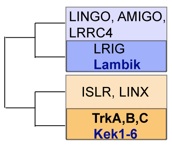
“Trks and Keks are more similar to each other than any other mammalian LIGs are to the Trks” based on the phylogeny by Mandai et al... Ginty(2009) Neuron 63, 614-627
Mammalian TrkB and truncated TrkB-T1; previous Drosophila ‘candidates’ Dror and Off-trk; and Drosophila LIGs.