Our research has been and is funded by:






Alicia Hidalgo lab School of Biosciences, University of Birmingham
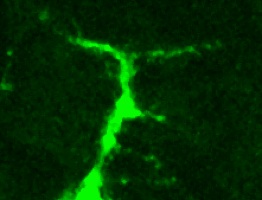

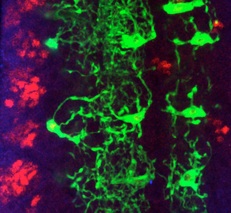
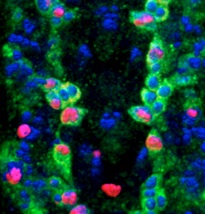
We investigate structural plasticity in the central nervous system (CNS), in health, in development, regeneration and repair. We use the fruit-fly Drosophila for its powerful genetics and in vivo neurobiology spanning from genes, to cells, circuits and behaviour. We discover gene networks, fundamental principles and in vivo processes with relevance for human brain health & disease.